Hardness removal is achieved in a single step with a low chemical cost using our CIF® (continuous ionic filtration) technology that combines physical filtration with ion exchange in a continuous and concurrent process.

Hard water is a term used to describe water with a high mineral content, usually referring to the concentration of multivalent cations. Calcium and magnesium ions are most commonly associated with causing hardness in water.
Hardness removal is important because hard water can cause:
- Scaling issues for pipes, turbines, boilers, and heat exchangers for mining, oil & gas, and industrial applications
- Altered taste in drinking water
- Creation of soap scum during cleaning
- Chemistry or efficiency issues when using hard water as process water
Hardness Removal Using CIF®
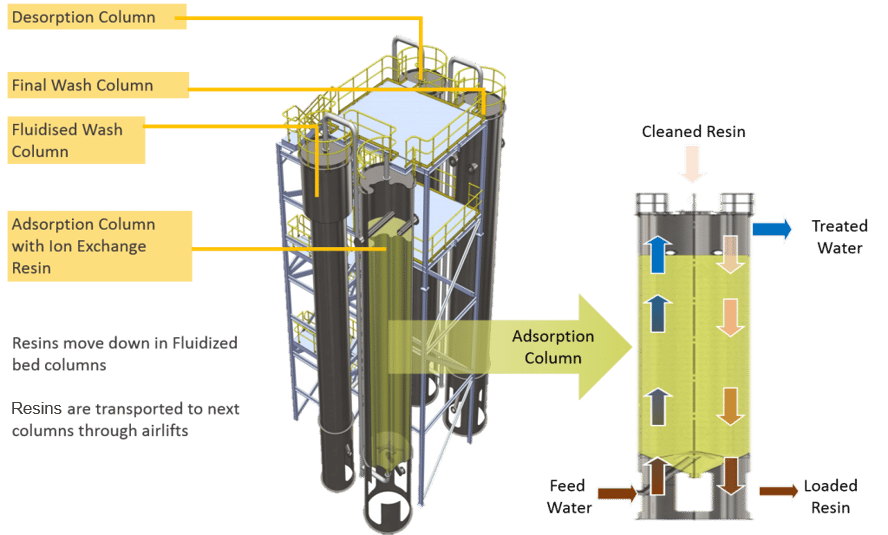
Our CIF® (continuous ionic filtration) technology combines physical filtration with ion exchange in a continuous and concurrent process, removing hardness to very low levels in a single step with a low chemical cost. In CIF®, ion exchange resins are moved between a series of four columns for resin washing and regeneration, providing continuous ion exchange. The packed bed of ion exchange resin also acts like a sand filter, reducing the suspended solids as water flows through the column.
The system is well suited to a range of industries, including:
- Largescale treatment of bore water for use as drinking water
- Reduction of hardness for mining/industrial process water or wastewater
- Hardness removal for water used in enhanced oil recovery (EOR) processes
The system offers the following benefits:
- Efficient hardness removal to very low levels due to the counter flow between water being treated and ion exchange resin
- Reduction of suspended solids as they are filtered using the packed resin bed
- Use of cheaper reagents such as sulphuric acid compared to batch ion exchange
- Minimal pre-treatment requirements
- Capable of reaching lower hardness levels than lime softening
- Robust water treatment, typically without scaling/fouling issues
- Zero liquid discharge capable in some applications
Clean TeQ Water can also provide flow sheets and equipment for softening very hard water (i.e. > 4,000 mg/L as CaCO3), and can provide solutions for selective hardness removal to allow recycling of process streams.

Hardness Removal Using Batch Ion Exchange
We also have experience using batch ion exchange for hardness removal to produce water suitable for drinking and process reuse. Batch ion exchange systems also achieve low hardness levels in treated water, but may require pre-treatment for solids and the use of hydrochloric acid for regeneration. Batch ion exchange systems can be economical when treating smaller water flowrates. Contact us to see which technology is best suited for the flowrate you are treating.
Benefits
EFFICIENT HARDNESS REMOVAL
TO VERY LOW LEVELS
ROBUST WATER TREATMENT
ZLD CAPABLE
LOW CAPITAL AND OPERATING COSTS
Get in touch
Find out how we can help with your water hardness removal challenge using the contact form below.






